About Oswald’s Pharmacy Use of the BD Veritor™ System for Rapid Detection of SARS-CoV-2
Introduction to Rapid COVID-19 Testing
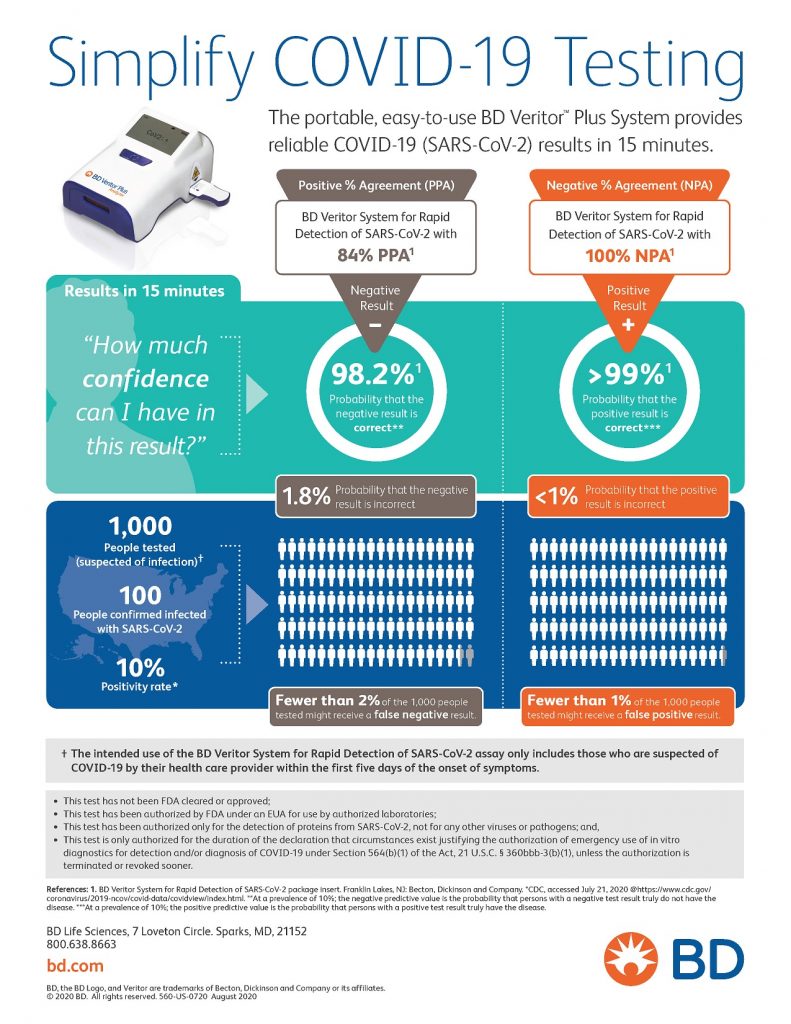
Information is taken from the manufacturer of our rapid COVID-19 Test, BD Medical.
The BD Veritor SARS-CoV-2 test is a chromatographic digital immunoassay intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid antigens in nasal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. In the USA, testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
Results are for the identification of the SARS-CoV-2 nucleocapsid antigen. This antigen is generally detectable in upper respiratory samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out a bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
What do your COVID-19 Test results mean?
If you test positive
Positive Test – Positive for the presence of SARS-CoV-2 antigen. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out a bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities (IDPH)
If you test negative
Negative Test – Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results be confirmed by a molecular testing method, if necessary, for patient management.
How Accurate is the Test?
There’s over a 99% accuracy for measuring positive test results. There’s a 98.2% accuracy for negative tests. That’s why they consider negative tests presumptive. Please follow all CDC guidelines on safety practices even after testing negative.
COVID-19 Test Warnings and Precautions
- For in vitro diagnostic use. In the USA, only for use under an Emergency Use Authorization.
- In the USA, this test has not been FDA cleared or approved; this test has been authorized by FDA under a EUA for use by authorized laboratories; use by laboratories certified under the CLIA, 42 U.S.C. §263a, that meet requirements to perform moderate, high, or waived complexity tests and at the Point of Care (POC), i.e., inpatient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and, in the USA, this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of the virus that causes COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1) unless the authorization is terminated or revoked sooner.
- Do not use this kit beyond the expiration date printed on the outside carton.
- Do not use the kit to evaluate patient specimens if either the positive control swab or negative control swab fails to give expected results.
- Test results are not meant to be visually determined. All test results must be determined using the BD Veritor Plus Analyzer.
- To avoid erroneous results, specimens must be processed as indicated in the assay procedure section.
- Do not reuse any BD Veritor System test device or kit components.
- When collecting a nasal swab sample, use the nasal swab supplied in the kit.
- Proper specimen collection, storage, and transport are critical to the performance of this test.
- Specific training or guidance is recommended if operators are not experienced with specimen collection and handling procedures. Wear protective clothing such as laboratory coats, disposable gloves, and eye protection when specimens are collected and evaluated.
- Pathogenic microorganisms, including hepatitis viruses and Human Immunodeficiency Virus, may be present in clinical specimens. Standard precautions and institutional guidelines should always be followed in the handling, storing, and disposing of all specimens and all items contaminated with blood or other body fluids.
- The SARS-CoV-2 positive control swabs have been prepared from recombinant viral proteins and do not contain infectious material.
- Dispose of used BD Veritor System test devices as biohazardous waste in accordance with federal, state, and local requirements.
- Reagents contain sodium azide, which is harmful if inhaled, swallowed, or exposed to the skin. Contact with acids produces a very toxic gas. If there is contact with the skin, wash immediately with plenty of water. Sodium azide may react with lead and copper plumbing to form highly explosive metal azides. On disposal, flush with a large volume of water to prevent azide build-up.
- Test devices used in a laminar flow hood or in areas with high airflow should be covered during test development to ensure proper sample flow.
- For additional information on hazard symbols, safety, handling, and disposal of the components within this kit, please refer to the Safety Data Sheet (SDS) located at bd.com.
FDA Link to the BD Rapid Test IFU
FDA Patient Fact Sheet for BD Rapid Test
CDC LINK ‘When to get Tested?’